Abstract
Introduction:
The use of ASCT is highly established as consolidation or salvage for multiple myeloma (MM) and post salvage therapy in non-Hodgkin Lymphomas (NHL), particularly diffuse large B-cell lymphoma (DLBCL) with chemosensitive disease, as well as upfront consolidation in PTCL given poor outcomes after standard therapy (Philip et al., NEJM 1995, Kewalramani et al., Br J Haematol 2006, Gisselbrecht et al., J Clin Oncol 2010, Kumar et al., Leukemia 2012). Although a significant number of pts experience long-term disease-free survival following ASCT, those with high-risk disease (i.e.high-risk cytogenetics in MM, primary refractory DLBCL) are likely to present early relapses, particularly in the first 18 months post-ASCT, illustrating the need for better disease control strategies following ASCT. The rapidly rising impact of checkpoint inhibitors in oncology provides an opportunity for its usage as post-ASCT consolidation, especially given the favorable immunologic milieu found in the immediate post-ASCT setting (i.e. decreased T-regs, increased effector T-cells) and minimal expected tumor burden at that time. Here, we report preliminary safety and efficacy data of a Phase I trial evaluating I and N as post-ASCT consolidation.
Methods:
Pts with the following malignancies were eligible, if they presented at least stable disease after most recent line of therapy:
DLBCL: primary refractory or relapsed,
PTCL: de novo stage III/IV or relapsed,
MM: transplant-naïve with high-risk cytogenetics or relapse within 3 years of upfront ASCT.
Pts were enrolled prior to ASCT, starting in July 2016. Total accrual goal is 42 patients. All pts with DLBCL/PTCL received BEAM (carmustine 300 mg/m2 day -6, etoposide 200 mg/m2 and cytarabine 200 mg/m2 days -5 to -2, melphalan 140 mg/m2 day -1) as conditioning regimen for ASCT, all pts with MM received melphalan 200 mg/m2 on day -1.
For pts who achieved appropriate hematologic recovery (ANC >800/mm3 and platelets > 20,000/mm3), I/N were started between days 14 and 28 post ASCT. The infusion schedule was:
• I: 1 mg/kg; 6 doses Weeks 1, 4, 7, 10, 16, 22
• N: 3 mg/kg; 12 doses Weeks 1, 4, 7, 10, 12, 14, 16, 18, 20, 22, 24, 26
At this time, 25 patients have been enrolled and received at least one dose of I/N. Additional pts are in screening and results will be updated.
Safety:
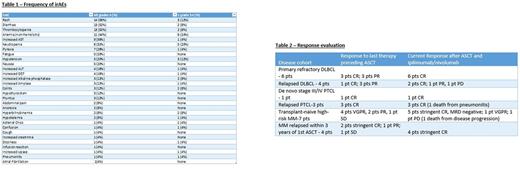
Median follow-up from time of first I/N infusion is 24 weeks (range 2-49). Adverse events (AEs) were documented starting week 1, day 1 of I/N infusion. AEs deemed at least possibly related to I and/or N were termed immune-related (irAEs) with 80% of pts developing irAEs of any grade (table 1). Treatment-related AEs of any grade that led to discontinuation of I/N occurred in 6 pts (24% total: colitis 12%, pneumonitis 4%, adrenal crisis 4% and hepatotoxicity 4%). One death attributable to I/N occurred (due to recurrent pneumonitis complicated by parainfluenza). Therapy with systemic steroids for management of irAEs was required for 19 pts (76%). 70% of irAEs improved within one week and 65% resolved within 2 weeks of initiation of steroids. Median time on treatment with I/N for development of irAEs was 9 weeks (range 2-25). For pts who discontinued treatment due to toxicity, the median time on I/N was 5 weeks (range 3-14). Incidence of irAEs was similar across disease groups.
Efficacy:
With a median follow-up of 24 weeks, OS is 92% and PFS is 88% for the entire cohort. 100% of the pts with relapsed MM after first ASCT (50% of whom had less than CR to 1st ASCT) are now in stringent complete remission (sCR). 100% of pts with primary refractory DLBCL are in CR (table 2).
Discussion:
The toxicity profile of consolidation with I/N following ASCT was within expectations. Although there has been a significant number of irAEs (80%) given the mechanism of action of these drugs, this rate is not higher than what has been previously reported with I/N combination in other disease settings (Larkin et al., NEJM 2015, Postow et al., NEJM 2015) and all patients except 1 had resolution of irAEs with the use of systemic steroids . With a median follow-up of 6 months, 84% of pts across disease groups are in complete remission. Interestingly, 5 of 6 patients who had early discontinuation due to AEs, presented sustained remission. Correlative studies evaluating blood immunophenotype are being reported in a separate abstract.
Skarbnik: Novartis: Speakers Bureau; Genentech: Speakers Bureau; Gilead: Speakers Bureau; Abbvie: Other: Ad board, Speakers Bureau; Seattle Genetics: Speakers Bureau. Goy: Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pharmacyclics / J&J: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Siegel: Merck: Consultancy; Celgene, Takeda, Amgen Inc, Novartis and BMS: Consultancy, Speakers Bureau. Biran: Takeda: Speakers Bureau; Celgene, Amgen: Consultancy, Speakers Bureau. Richter: Janssen: Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Feldman: Kite Pharma: Speakers Bureau; Seattle Genetics: Honoraria, Research Funding, Speakers Bureau; Celgene: Speakers Bureau; Pharmacyclics: Speakers Bureau; Janssen: Speakers Bureau; Bristol-Myers Squibb: Consultancy; AbbVie: Speakers Bureau. Leslie: seattle genetics: Speakers Bureau; KITE pharma: Speakers Bureau; celgene: Speakers Bureau. McKiernan: Novartis: Speakers Bureau. McNeill: pharmacyclics: Speakers Bureau; celgene: Speakers Bureau; seattle genetics: Speakers Bureau. Pecora: Caladrius Biosciences: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; COTA: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal